QLS‑111 is a molecule being investigated for
its role in lowering IOP.
QLS‑111 is a novel topical formulation using
Qlaris Bio’s ATP-sensitive
potassium channel modulator platform originally developed by Dr. Michael
Fautsch, PhD, professor of ophthalmology, biochemistry, and molecular biology
at Mayo Clinic, in Rochester, Minnesota.
QLS-111 lowers IOP by relaxing vessels of
the vascular and vascular-like tissues distal to the trabecular meshwork,
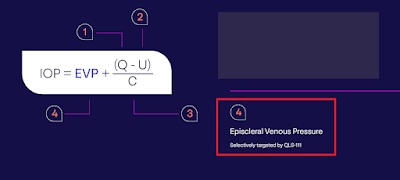
thereby reducing distal outflow resistance and lowering episcleral venous pressure (EVP).
Thus, it is potentially suitable for
treating primary open-angle glaucoma (POAG), ocular hypertension (OHT), and
normal tension glaucoma (NTG).
EVP contributes up to 50% of total IOP. If
unchecked, EVP may play a role in the inability to reach target IOP. Therefore,
QLS‑111's complementary mechanism may offer a
significant and synergistic advantage in managing IOP.
QLS-111 has demonstrated that it can work
alone or in combination with several current glaucoma drug classes, indicating
that its use with current medications will aid in the ability to achieve levels
of IOP reduction that slow disease progression.
Qlaris Bio, Inc. (“Qlaris”), a
clinical-stage biotechnology company targeting unmet needs in debilitating
ophthalmic diseases, announced the initiation and dosing of two separate
U.S. Phase II masked, randomized clinical trials investigating QLS‑111 in patients with ocular hypertension and glaucoma.
The company is initiating the following
studies:
The Osprey study (NCT06016972) will assess
the safety, tolerability, and optimal dose of QLS‑111
compared with vehicle alone in adult patients who have POAG and/or OHT. The efficacy of QLS-111 in
lowering IOP will be assessed as a secondary endpoint of the trial.
The Apteryx study (NCT06249152) will
evaluate the safety and tolerability, and measure the additive IOP-lowering
efficacy, of QLS‑111 in combination with latanoprost versus
latanoprost alone. Patients aged 12 years or older and who have POAG and/or
OHT currently on latanoprost will be enrolled in the trial.
A few studies have demonstrated that
treatment with QLS‑111 provides persistent lowering of IOP,
maintains normal vascular integrity of the venous system, is well-tolerated,
and thus far has shown it does not cause hyperemia. [Information from company website]









No comments:
Post a Comment