The Eyemate system consists of a permanent
implantable and biocompatible micro sensor, which provides continuous
intraocular pressure (IOP) measurements. The Eyemate-SC, developed by the German
company Implandata Ophthalmic Products, is inserted into the suprachoroidal
space. The Eyemate-IO is implanted in the ciliary sulcus.
Sulcus implanted wireless telemetric
sensors were shown to be generally safe and to provide long-term reliable IOP
measurements. However, ciliary sulcus implantation has numerous disadvantages
such as iris chafing and atrophy, pupillary distortion and pigment dispersion.
The implants are reserved for pseudophakic or cataract patients, which excludes
younger patients for whom long-term IOP monitoring is particularly important. Furthermore,
the bulky sensor ring needs a large corneo-scleral incision and causes
excessive manipulation during sulcus implantation. The ARGOS and ARGOS-02
trials for the Eyemate-IO did show good patient tolerability and provided
measurements that closely agreed with manometric pressure measurements.
The Eyemate device consists of eight
pressure- and temperature-sensitive capacitors attached to a gold circular
antenna, and readings are transmitted through an external handheld device which
also charges the Eyemate through electromagnetic coupling.
The IOP measurements are transmitted via a
wireless data connection to a secure internet-based database. This enables the
treating physician to monitor the IOP and then decide the adjustment of the
glaucoma medications.
The Eyemate smartphone app will provide
helpful information about the disease status and the therapy success to the
patient and a medication schedule can be established, which reminds the patient
automatically when he/her has to apply his/her glaucoma medication.
One of the main advantages of IOP sensors
is that they are independent of corneal biomechanics. While corneal-surface-based
tonometry techniques only measure relative dimensional changes of the eye with
questionable validity, intraocular sensors directly measure the absolute IOP.
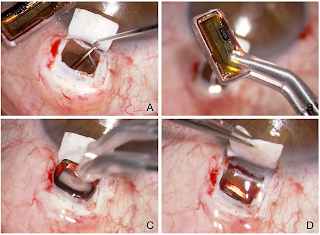
SURGICAL TECHNIQUE:
To implant the sensor, the suprachoroidal
space is accessed either through the window (100% thickness technique) or
through a 5mm incision in the remaining scleral lamella (90% thickness
technique). The choroid is separated from the sclera by means of OVD, and the
EYEMATE-SC implant is carefully inserted into the suprachoroidal space using
padded implantation forceps and avoiding contact with the sensor’s ASIC.
Afterward, the superficial scleral flap and the conjunctiva are closed and
sutured.
The most frequent postoperative
complication after Eyemate implantation is hyphema. Other complications
reported are: superficial punctate keratitis, postoperative leakage, choroidal
detachment and hypotony, postoperative photopsia, touch sensitivity, slight
pain in the operative area, and intermittent headaches. No serious AEs related
to the EYEMATE-SC sensor have been reported.
REFERENCES:
- Szurman P, Gillmann K, Seuthe AM, Dick HB, Hoffmann EM, Mermoud A, Mackert MJ, Weinreb RN, Rao HL, Mansouri K; EYEMATE-SC Study Group. EYEMATE-SC Trial: Twelve-Month Safety, Performance, and Accuracy of a Suprachoroidal Sensor for Telemetric Measurement of Intraocular Pressure. Ophthalmology. 2023 Mar;130(3):304-312. doi: 10.1016/j.ophtha.2022.09.021. Epub 2022 Oct 3. PMID: 36202141.
- Szurman P, Mansouri K, Dick HB, Mermoud A, Hoffmann EM, Mackert M, Weinreb RN, Rao HL, Seuthe AM; EYEMATE-SC study group. Safety and performance of a suprachoroidal sensor for telemetric measurement of intraocular pressure in the EYEMATE-SC trial. Br J Ophthalmol. 2023 Apr;107(4):518-524. doi: 10.1136/bjophthalmol-2021-320023. Epub 2021 Nov 12. PMID: 34772665; PMCID: PMC10086291.
- Koutsonas A, Walter P, Roessler G, Plange N. Implantation of a novel telemetric intraocular pressure sensor in patients with glaucoma (ARGOS study): 1-year results. Invest Ophthalmol Vis Sci. 2015 Jan 22;56(2):1063-9. doi: 10.1167/iovs.14-14925. PMID: 25613949.
- Choritz L, Mansouri K, van den Bosch J, Weigel M, Dick HB, Wagner M, Thieme H; ARGOS study group. Telemetric Measurement of Intraocular Pressure via an Implantable Pressure Sensor-12-Month Results from the ARGOS-02 Trial. Am J Ophthalmol. 2020 Jan;209:187-196. doi: 10.1016/j.ajo.2019.09.011. Epub 2019 Sep 20. PMID: 31545953.











No comments:
Post a Comment