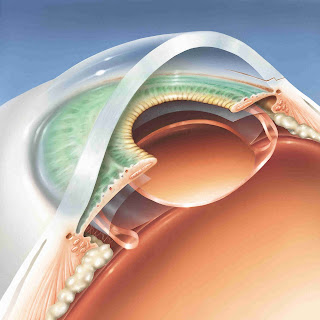
The eyeWatch glaucoma device
Glaucoma is a multifactorial disease
characterized by progressive damage to the retinal ganglion cells (RGCs) and
its axons. The management of this condition is presently restricted to control
of intraocular pressure (IOP) to such levels that progression can be reduced or
even halted. Management consists of pharmacological methods, laser procedures,
and surgery. Conventionally, surgery was restricted to some type of filtering
procedure which allowed aqueous to drain from the eye. However, trabeculectomy
failure rates can be a significant problem in certain series of patients. Over
the last few years, glaucoma drainage devices (GDDs) have been introduced to
overcome the myriad complications seen with trabeculectomy. However, these
methods too are far from successful in all patients.
One of the major problems with glaucoma
filtering surgeries and also GDDs is that of hypotony which occurs in
the immediate post-operative period. This occurs either due to over-filtration
or possibly the ciliary shock mechanism, which causes less aqueous humor to be
produced in the early post-operative phase. While this problem is not so
significant in trabeculectomy, as surgeons have found methods to control that
by releasable sutures, padding the eye and so on, this complication is more
significant following the use of GDDs. Some GDDs have a rip-cord in their
drainage tube which can be removed after a few days of surgery. Others employ
sutural ligation of the tube to prevent early postoperative hypotony. The
drawbacks of such flow-restricting techniques are the lack of precision and
predictability in efficiently controlling IOP in the early postoperative phase,
which may result in volatile IOP with initial hypertensive and subsequent
hypotensive phase.
Recently, a new method to control aqueous
drainage following the implantation of GDDs has been developed in Switzerland.
This device is known as the eyeWatch. Developed by Rheon Medical, Lausanne,
Switzerland, the device can adjust the aqueous outflow and thus, better control
IOP. The device consists of a deformable silicone tube which connects the
anterior chamber to any GDD. Rheon Medical also has an in-house plate valve known
as the eyePlate.
The tube is compressed by rotating a
magnetic disc on a shaft eccentric to its axis of symmetry. This compression
makes changes in the cross-sectional area of the tube and thus, changes the
fluidic resistance. This in turn, alters the aqueous outflow through the
anterior chamber. The magnetic disc is adjusted by a non-invasive external control unit
known as the eyeWatch Pen. The Pen is kept 1-2 mm from the device and dialed from
0 to 6, indicating fully open to fully closed respectively.
The steps in the surgical procedure to
implant eyeWatch are available at the following website:
In short, a GDD is first sutured to the sclera. A bed in the sclera is created using the eyeWatch marker. Subsequently, a 25-G needle is used to enter the anterior chamber. An anterior chamber maintainer is useful. The nozzle of the eyeWatch is stiff and short. This avoids damage to the corneal endothelium. The eyeWatch is sutured to the sclera. Subsequently, the tube of the GDD is cut to an appropriate length and attached to the eyeWatch. Finally, a 7mmx7mm pericardial patch graft is sutured to the sclera to cover the device and prevent tube erosion. No anti-metabolites are required in this surgery.
In a study of 15 patients implanted with
the eyeWatch, mean baseline pre-operative IOP of 26.2±6.8mmHg reduced to
11.9±2.8mmHg at 12 months (P<0.001). The mean number of glaucoma medications
decreased from 3.0 ±0.7 before surgery to 0.8 ±0.9 at last visit (P<0.001). The
success rate was 40% for complete success (IOP between 6-18 mmHg, with a 20%
reduction from baseline, without medications) and 93% for overall success at
last follow-up. Complication rate was 7%. The complications affecting 4
patients included 2 cases of conjunctival wound leak (Seidel sign positive) and
2 cases of choroidal detachment.
A few questions are raised regarding the
procedure. One, is the magnet affected during MRI? The study mentioned above did not
find any interference of the imaging technique by eyeWatch. Although, all
patients (n=4) who underwent MRI required readjustment of the eyeWatch
settings following the MRI. The second question is that the device does not address the main
causes of GDD failure, that is, fibrosis around the implant. Another question
which can be asked is whether the presence of a magnet inside the human eye
would affect the physiology of not just the eye, but also the body. There are
conflicting reports regarding the ability of the human body to sense magnetic
fields. Birds and bats are classic examples of using the earth’s magnetic field
to navigate. Although humans probably do not need those cues for their sense of
direction, the long term effects of such devices in the eye are unknown. It
should also be cautioned that GDDs are often used in patients with advanced
glaucoma, a group in whom vision and visual fields are already compromised.
Long-term results of this device shall be awaited by glaucoma surgeons, as this could prove to be an important development in IOP adjustment following successful glaucoma surgery.