ROCK-inhibitors
Rho-associated coil-forming
protein kinases (ROCK):
Not since 1996, the year in which
FDA had approved Latanoprost, has another class of glaucoma drugs been
marketed. However, now there is a distinct possibility that drugs which improve
outflow through the trabecular meshwork could be approved. Among those agents
which enhance aqueous outflow through the conventional pathway (so-called “pharmacologic
trabeculectomy”), the ROCK-inhibitors show the most promise.
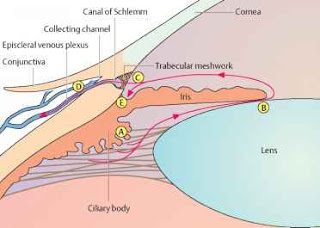
The trabecular meshwork cells
exhibit a smooth-muscle like phenotype based on their expression of various
smooth muscle specific proteins including α-smooth muscle actin (α-SMA)
and CPI-17 (Protein kinase C-potentiated protein phosphatase-1 inhibitor
protein). Numerous microfilament-based
structures are also found in cells of the outflow pathway. These include focal
contacts, adherens cell-cell junctions and bundles of microfilaments.
The regulation of trabecular
meshwork contractility is under calcium-dependent and calcium-independent
mechanisms. Smooth muscle contraction is predominantly regulated by the
phosphorylation of Myosin Light Chain (MLC). MLC is phosphorylated by
calcium/calmodulin-dependent MLC kinase (MLCK) and dephosphorylated by
calcium-independent MLC phosphatase (MLCP). Apart from Ca++ concentration, MLC
phosphorylation can be modulated via signaling pathways such as the Rho/Rho
kinase pathway.
The trabecular meshwork and
ciliary muscle are known to express many components of the Rho signaling
pathway such as ROCK1 & ROCK2, RhoA, MLC, MLCK and MLCP. ROCK activity
through the Rho signaling pathway is thought to be a key player in regulating
the cellular morphology and contractility of the conventional outflow pathway.
The Rho family consists of 3
small guanosine-triphosphate (GTP) binding proteins RhoA, RhoB, and RhoC. These
proteins regulate cell shape, motility, proliferation, and apoptosis throughout
the body. Rho binds to GTP, activating its downstream effector molecules (ROCK1
and ROCK2).
The Rho-associated coil-forming
protein kinases (ROCK) are protein serine/threonine kinases. They occur in 2
isoforms: ROCK1 and ROCK2. The former is located on chromosome 18 and contains
1354 aminoacids. The latter is located on chromosome 12 and encodes a 1388
aminoacid product. In humans ROCK1 and ROCK2 are expressed in majority of
tissues, including the trabecular meshwork and ciliary muscle cells.
Structurally, ROCKs are composed
of 3 major domains:
- An N-terminal kinase domain (which phosphorylates protein targets)
- A C-terminal autoinhibitory domain (which limits kinase activity via intramolecular interactions)
- Coiled-coil Rho-binding domain (which facilitates the switch from the inactive to active conformation)
The 2 isoforms of ROCK are the
downstream targets of the small GTP-binding protein: Rho. The Rho GTPases act
as molecular switches by cycling between an active GTP-bound and an inactive
GDP-bound form. On binding to Rho, the catalytic activity of ROCKs is
moderately enhanced. ROCKs mediate a number of important cellular functions
such as cell shape, motility, secretion, proliferation and gene expression.
ROCKs also mediate RhoA-induced actin cytoskeletal changes by inhibiting MLCP.
These directly affect the contractile properties of the trabecular meshwork
outflow tissues. ROCKs also activate LIM-kinases which stabilize filamentous
actin to reduce the occurrence of cell migration.
ROCKs have some distinct
functions in the region of the trabecular meshwork:
- Regulation of the movement and shape of cells through its action on the cellular cytoskeleton.
- Contribution to abnormal accumulation of extra-cellular material (ECM hypothesis). Anterior chamber perfusion with metalloproteinases, which digests ECM is found to improve outflow.
- Changes in the contractile activity and cell adhesive interactions of the cells of aqueous outflow pathway (Contractility hypothesis). Experimental disruption of the actin cytoskeleton of the trabecular meshwork decreases outflow resistance, while the trabecular meshwork of patients with primary open angle glaucoma is stiffer than that of age-matched controls, contributing to the contractility hypothesis.
It is hypothesized that there is
an increased expression of Rho/ROCK pathway in the outflow tissues in
glaucomatous eyes. These actions of ROCK lead to increased resistance to
aqueous humor outflow through the trabecular meshwork.
ROCK-inhibitors:
ROCK-inhibitors induce reversible
modifications to cell morphology and cell interactions in the eye that
facilitate greater outflow of aqueous humor through the trabecular meshwork and
ultimately lower the intra-ocular-pressure (IOP). ROCK-inhibitors uncouple
actin from myosin, 2 proteins which interact to contract the ciliary muscle. Thus,
specific components of the cellular cytoskeleton are disrupted, reducing the
contractile tone of the tissues of the conventional outflow pathway. By
inhibiting Rho-kinase actin-myosin contractility, it also allows the cells to
relax. This creates space between the cells through which fluid can exit from
the eye.
ROCK-inhibitors also increase
ocular blood flow in the optic nerve head by relaxation of the vascular
endothelial smooth muscle. Nitric oxide induced impairment of optic nerve blood
flow was reportedly prevented by the ROCK-inhibitor Fasudil.
ROCK-inhibitors also have a
vasodilatory effect, which may lead to reduced episcleral venous pressure.
ROCK-inhibitors have been found
to influence neuron survival and axon regeneration. In a study, Fasudil
protected against glutamate-related excitotoxicity in the retina and better
preserved cells of the ganglion cell layer on exposure to N-methyl-D-aspartate.
ROCK-inhibitors block TGF-β
myofibroblast transdifferentiation of human tendon fibroblasts which suggests
that ROCK-inhibitors may reduce postoperative scarring after glaucoma filtering
surgery.
Side effects of
ROCK-inhibitors:
Side effects of ROCK-inhibitors
include mild conjunctival hyperemia, which spontaneously resolves over several
hours. This is assumed to be due to the vasodilatory effect of the drug. It is
seen in 50-60% of the treated individuals. By instilling the drug at night,
there could be a symptomatic decrease in the frequency of this side effect.
Small conjunctival hemorrhages and cornea verticillata (seen in patients who
are on concurrent systemic amiodarone) are also seen. These features are
asymptomatic and do not reduce visual function.
Higher concentrations of
ROCK-inhibitors may affect other protein kinases in the body including Protein
kinase A, Protein kinase C and MLCK among others. ROCK-inhibitors lower blood
pressure and reduce vascular resistance. This could be detrimental in the
elderly.
ROCK inhibition was also found to
reduce the intraocular penetration of concurrent Timolol instillation
(presumably by increasing the elimination through dilated conjunctival
vasculature).
However, no significant systemic
side-effects have been reported with these agents.
Rhopressa, Ripasudil and
Roclatan:
Although a number of
ROCK-inhibitors were studied, yet only few have reached Phase 3 trials. Among
them Netasurdil, Roclatan and Ripasudil are prominent. The last is available
only in Japan.
Netarsudil 0.02% (Rhopressa)
lowers IOP by inhibiting ROCK and the norepinephrine transporter (NET). The
former action enhances trabecular outflow and reduces episcleral venous
pressure; while through NET it decreases aqueous production. Conversely, Ripasudil,
is purely a ROCK-inhibitor.
Roclatan (PG-324) is a once-daily
eyedrop which combines a fixed dose Netasurdil 0.02%(Rhopressa, AR-13324) with
Latanoprost 0.005%.
Roclatan theoretically lowers IOP
through the following mechanisms:
- Increasing aqueous outflow through the trabecular meshwork.
- Increasing aqueous outflow through the uveo-scleral pathway
- Reducing aqueous production.
- Reducing episcleral venous pressure.
Phase 1,2, and 3 clinical trials
of Netarsudil were conducted on more than 2000 patients. 0.02% once daily in the
evening was found to be the most efficacious and well tolerated dosing regime.
The phase 2 clinical trial included comparison with Latanoprost. IOP reductions
in both groups were similar (-5.8 mmHg in Netarsudil vs 5.9 mmHg in the
Latanoprost group). Among all patients Netarsudil was 1 mmHg less effective
than Latanoprost (-5.7 vs 6.8 mmHg) and did not meet the statistical analysis
for noninferiority of Netarsudil to Latanoprost.
Rocket 1,2,4 phase 3 trials
compared Netarsudil to Timolol. All studies showed comparable IOP reduction
with both drugs.
Mercury 1 and 2 have shown 1-3
mmHg greater IOP lowering with Roclatan compared to the individual components
(Netarsudil and Latanoprost). IOP reductions of atleast 30% were achieved in 65%
of patients treated with Roclatan, compared to 40% when individual components
were used.
In phase III, Mercury 2 trials, Roclatan
achieved successful efficacy results. Roclatan treated patients achieved 16
mmHg or less IOP in 61% cases, while ≤14 mmHg was achieved in 33% cases.
This was comparable to those treated with individual components in whom similar
results were achieved in 40% or less and 15% or less respectively. The product
was well tolerated with a 10% discontinuation rate.
Conclusion:
The advent of new drugs, which modulate aqueous outflow through the trabecular meshwork, which is a more physiological route, might have a positive correlation to IOP control in glaucoma/Ocular hypertension patients. According to one theory, by giving prostaglandin-analogues, we increase uveo-scleral outflow and further reduce the activity of the conventional outflow pathway. Thus, ROCK-inhibitors may prove to be an important milestone in glaucoma management.