OPTIC ATROPHY
DEFINITION: Optic atrophy (OA) is a pathological
term referring to optic nerve shrinkage caused by degeneration of retinal
ganglion cell (RGC) axons. Optic atrophy is somewhat of a misnomer as atrophy
implies disuse. Therefore, OA is better termed “optic neuropathy”.
INTRODUCTION: OA is a non-specific sign of a
disease process affecting the visual pathway. In
adults it implies the retinogeniculate portion of the visual pathway. While the
peripheral nervous system has an intrinsic ability for repair and regeneration,
the central nervous system, for the most part, is incapable of such processes.
The axons of the optic nerve are heavily myelinated by oligodendrocytes, and
the axons, once damaged, do not regenerate. Thus, the optic nerve behaves more
like a white matter tract rather than a true peripheral nerve. The optic nerve
head (ONH) is supplied by pial capillaries which undergo degeneration leading
to pallor of the ONH in OA.
When
light is thrown on the fundus from a light source it undergoes total internal
reflection through the axonal fibers. Subsequently, reflection from the
capillaries on the disc surface gives rise to the characteristic yellow-pink
color of a healthy optic disc. However, the degenerated axons in OA lose this
optical property leading to the pale optic disc seen in this condition.
Although
glaucoma is also a cause of OA, it is regarded as a distinct entity.
Clinically, non-glaucomatous OA is usually recognized by a diffuse or
segmental pallor of the optic disc. Conversely, glaucomatous OA is
characterized by cupping and a preserved healthy neuro-retinal rim until
advanced disease. Management of OA is directed at determining the cause and
directing treatment at the etiologic process. Non-specific management might be
required in cases which do not respond to the specific treatment.
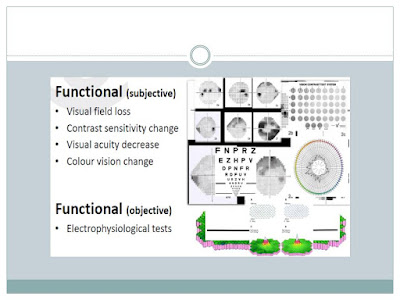
CLINICAL FEATURES: Patients may complain of loss of
vision with segmental or diffuse blurring of the visual field. There can be
reduced color saturation or contrast sensitivity. The normal linear association
between stereoacuity and Snellen visual acuity could also be lost in OA.
TYPES: OA can be classified in different manners.
This
can be in the form of Primary/secondary/consecutive or depending on the
etiology.
“Primary
OA” occurs without any antecedent ONH swelling. It is caused by lesions
involving any part of the visual pathway extending from the retrobulbar portion
of the optic nerve to the lateral geniculate body. It is seen in conditions such as pituitary or
optic nerve tumors and aneurysms, hereditary- and traumatic- optic
neuropathies, toxic- and nutritional-optic neuropathies and multiple sclerosis
and following retrobulbar neuritis. The optic nerve fibers degenerate in an
orderly manner and get replaced by columns of glial cells without alteration in
the architecture of the ONH. The disc is pale, chalky white with sharply defined
margins and the parapapillary blood vessels attenuated. Lamina cribrosa is well
defined. Thinning of the RNFL is present with often a reduction in the number
of small blood vessels on the optic disc surface (Kestenbaum sign= normally the
number of capillaries on the ONH is 10. But in OA the count may reduce to 6).
“Secondary
OA” follows optic disc swelling (papilledema, optic neuritis, anterior ischemic
optic neuropathy). Optic nerve fibers exhibit marked degeneration, with
excessive proliferation of glial tissue. The ONH architecture is lost,
resulting in indistinct margins. The disc is grey or dirty-grey; the margins
are poorly defined and slightly raised, with the lamina cribrosa obscured due
to proliferating fibroglial tissue. Hyaline bodies (corpora amylacea) or drusen
may be observed. Peripapillary sheathing of arteries as well as tortuous veins
may be observed. Progressive contraction of visual fields is usually seen.
“Consecutive
OA” occurs in diseases affecting the inner retina or it’s blood supply. It can
be seen in: retinitis pigmentosa, pathological
myopia, following pan-retinal photocoagulation, extensive retinochoroiditis and
central retinal artery occlusion. The ONH is waxy pale with a normal disc
margin, marked attenuation of arteries and a normal physiologic cup.
INVESTIGATIONS:
- Visual fields 30-2 and full field. In optic neuropathy, visual field changes can include enlargement of the blind spot and paracentral scotoma (eg, optic neuropathy), altitudinal defects (eg, anterior ischemic optic neuropathy, optic neuritis), and bitemporal defects (eg, compressive lesions, similar to optic chiasma tumors).
- MRI of the brain and orbits with contrast (in addition to space-occupying lesion [SOL], look for sinusitis, hyperpneumatized sinuses, fibrous dysplasia)
- Blood glucose level
- Blood pressure, cardiovascular examination
- Carotid Doppler ultrasound study
- Vitamin B-12 levels
- Venereal Disease Research Laboratory (VDRL)/Treponema pallidum hemagglutination (TPHA) tests
- Antinuclear antibody levels
- Sarcoid examination
- Homocysteine levels
- Antiphospholipid antibodies
- Enzyme-linked immunosorbent assay (ELISA) for toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus (TORCH panel)
Certain
specific imaging studies are required to assess the cause of OA. These include:
- For tumors located in the orbit, ultrasonography is recommended.
- For papilledema, a B-scan ultrasound is recommended to look for optic sheath dilatation.
- To find out whether a lesion is cystic or solid (eg, cysticercoids), CT or MRI is recommended. For solid lesions, MRI (with contrast or fat suppression) is preferred in areas in close proximity to the bony wall.
- For fractures associated with trauma, a noncontrast CT scan is preferred.
- For multiple sclerosis, a gadolinium-enhanced MRI/fluid-attenuated inversion recovery (FLAIR) sequence is useful to detect hyperechoic areas.
- Subnormal: Potential less than 0.08 microvolts; seen in toxic neuropathy
- Negative: When a large a-wave is seen; may be due to giant cell arteritis, central retinal artery occlusion, or central retinal vein occlusion
- Extinguished: Response seen in complete optic atrophy
Visually evoked
response (VER):
In optic neuritis,
the VER has an increased latency period and decreased amplitude as compared to
the normal eye.
Compressive
optic lesions tend to reduce the amplitude of the VER, while producing a
minimal shift in the latency.
TREATMENT:
There is no proven treatment to reverse OA. However, treatment of the exact
causative factor prior to significant damage might be helpful in saving useful
vision. Low vision aids (LVA) may also be of benefit in selected cases.
In optic neuritis, arteritic
anterior ischemic optic neuropathy and traumatic optic neuropathy intravenous
methylprednisolone has been found beneficial.
Idebenone, a quinone
analog, is the only clinically proven drug in the treatment of Leber hereditary
optic neuropathy.
Stem cell treatment may
prove to be a key in the future treatment of neuronal disorders.